Accelerating Insulin Production in North Carolina
Summary
We successfully delivered one of the largest insulin manufacturing facility expansions ever undertaken by the client. Our group of companies, recognised for our expertise in designing, building, and shipping large-scale stainless steel tanks, managed every aspect of the project. This included overseeing the transportation and on-site commissioning, which improved efficiency, reduced costs, and ensured the availability of essential diabetes medicines for patients worldwide.
Scope
The client required nine Pre-Assembled Units (PAUs) for diabetes (Insulin) medicines for a new production facility in North Carolina, USA, driven by the increasing need for more insulin medicines. As one of the largest tank and process manufacturers, Briggs of Burton (BRIGGS) was selected to deliver this project alongside sister company Ziemann Holvrieka.
Challenges
- Creating efficient and high-quality PAUs at scale that meet the stringent validation requirements for pharmaceutical production
- Ensuring compliance with safety standards, precision, and reliability
- Managing the scale of production to design, build and deliver them from Europe to North America
Solution
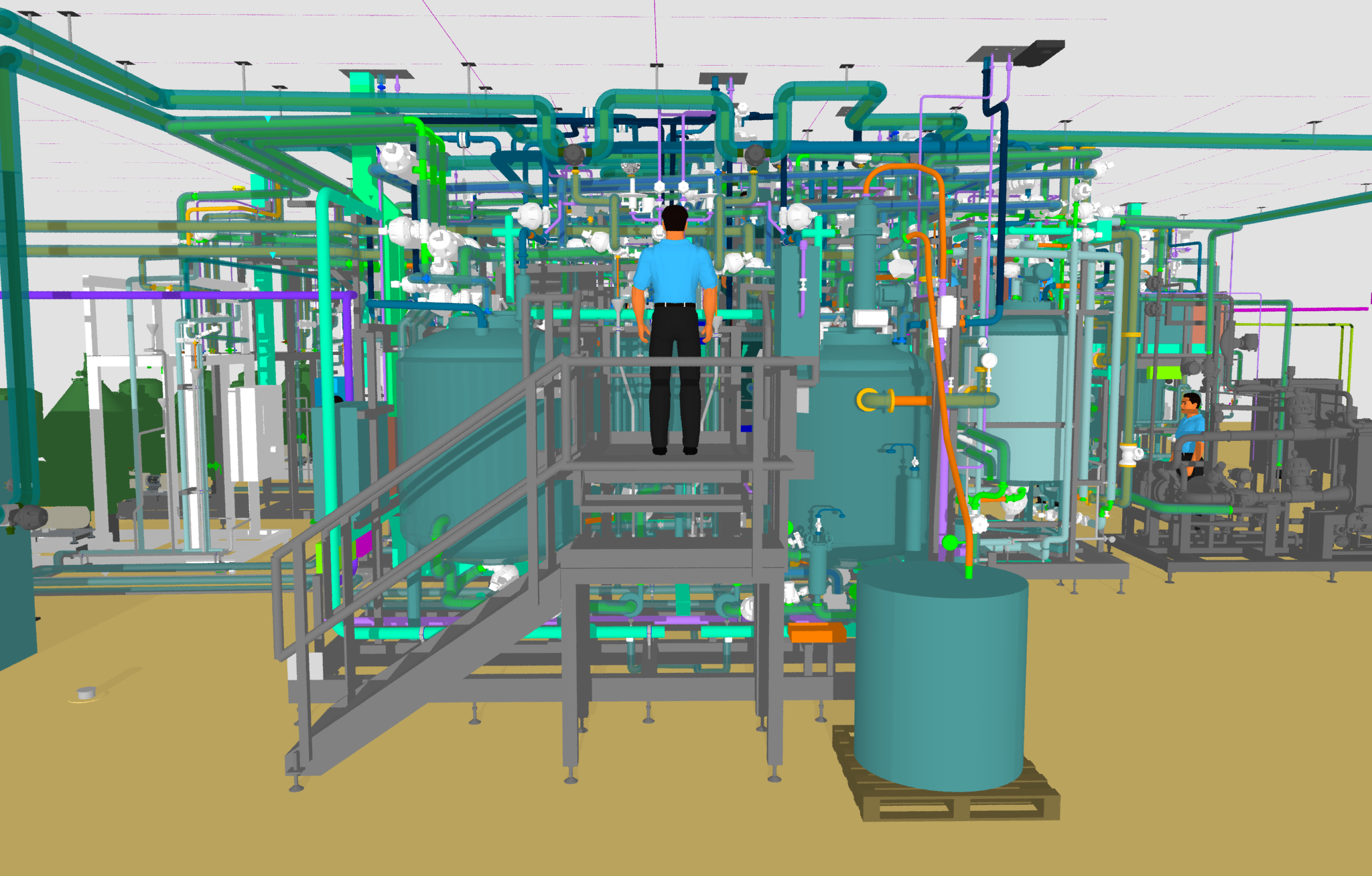
From design to delivery, our specialists coordinated the entire project. The pre-engineering team used advanced 3D modelling to design PAUs, including skids, pipework, and electrical components based on initial concepts and specifications. We created detailed Process and Instrument Diagrams (P&IDs) for process flow integration and compiled equipment schedules specifying materials and dimensions for each vessel.
Using other manufacturing companies in our Group, we ensure the successful fabrication and delivery of nine PAUs. To fabricate 28 pharmaceutical vessels, we used 316L stainless steel, Duplex, or Super Duplex. Our team manufactured storage tanks for pharmaceutical ingredients, tanks for precise solvent preparation, and chromatography tanks for efficient separation and purification processes. The vessels were built into units varying in diameter and height, ranging from one metre to ten metres.
We have expertise across our group not only to design and build these units but also to coordinate the logistics to ship them to their final location. We supervised on-site assembly and commissioning of the systems, guaranteeing the seamless integration of all PAUs into the new production facility.
Impact
The successful delivery of these PAUs significantly contributes to the client’s state-of-the-art pharmaceutical production facility. By streamlining the manufacturing process, the project enhances efficiency, reduces costs, and ensures the availability of critical diabetes medicines for patients worldwide. This project represents the largest manufacturing facility expansion undertaken by the client, valued at $2 billion.
Find out more: Pharmaceutical Systems Assembly (PSA) Facility
Pathway to a sustainable future: Sustainable process engineering | Briggs | Briggs (briggsplc.com)